Introduction
3D printing process allows to realize complex objects through a simple process due to its ease of use, adaptability of the system and low costs. 3D printing has found a great development in biomedical and tissue engineering fields for scaffolds production made with biocompatible thermoplastic polymers or high viscosity materials such as hydrogels.
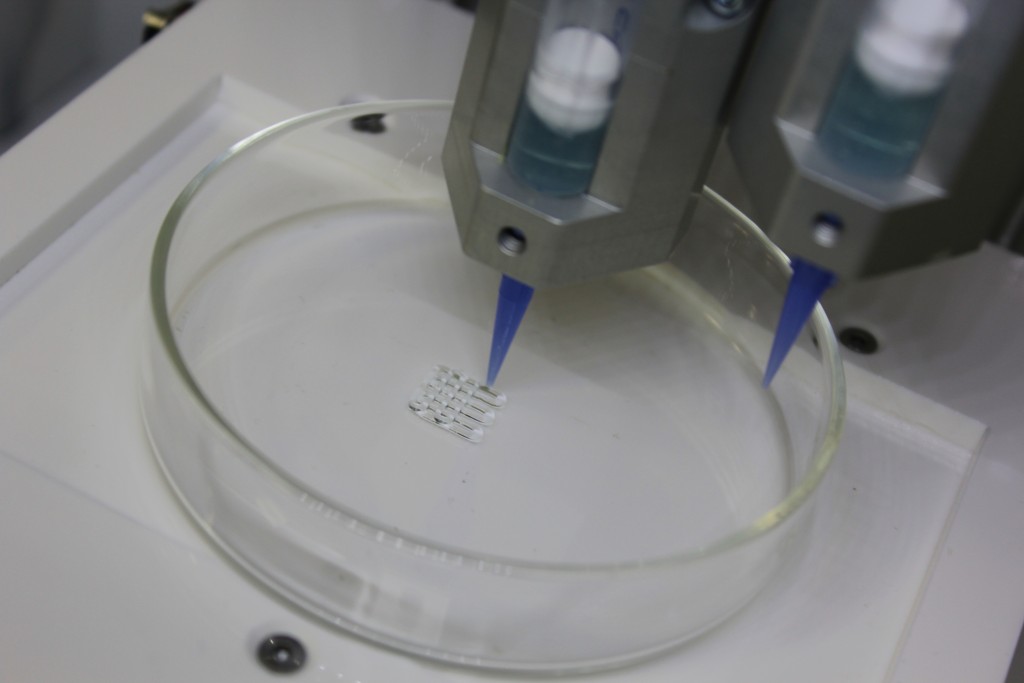
In the compounds, it is possible to encapsulate cellular components such as cells, growth factors and drugs. This process, called bioprinting [1], consists in three basic elements: 3D bioplotter (the extrusion-based system), bioink (drops of single cells or cell aggregates to be printed) and biopaper (the basis for depositing, layer by layer, the bioink). The CellInk INKREDIBLE+ is based on pneumatic extrusion, used to extrude hydrogel bioinks used to encapsulate cells for bioprinting 3D cell constucts to realize new cell models for biological applications.
Goal
ACTIVITY 1: 3D model muscle fiber(in collaboration with Prof. G. Cusella, Doc. G. Ceccarelli and Doc. F. Ronzoni – Anatomy Dept. of UniPV). 3D bioprinting mouse myoblast cell line to study cells differentiation into Cellink Fibrin bio-ink and 3D modelling muscle fiber physiology. Cells differentiate into 3D construct.
ACTIVITY 2: Bioprinting of 3D cell constructs for neurological studies (in collaboration with Prof. Cereda – Ist. Casimiro Mondino of Pavia). To develop a neural cell model for biological applications and disease studies using an alginate-based hydrogel.
ACTIVITY 3: Evaluation of novel bio-ink based on chitosan (in collaboration with Prof. B. Conti and Prof D. Pasini – Chemical Dept. of UniPV). To use Cellink INKREDIBLE+ for printing natural chitosan-based hydrogel combined for tissue regeneration.
ACTIVITY 4: Cell modelling (in collaboration with Doc. C. Ferrari, Doc. L. Consolino – Experimental Surgery Lab. of UniPV). 3D printing osteosarcoma cell line encapsulated into alginate and gelatin hydrogel for cancer model.
ACTIVITY 5: 3D printing thermoplastic material (Progetto Ricerca Corrente 2018, IRCCS Policlinico San Matteo, PV). To use Cellink INKREDIBLE+ for 3D-printing polycaprolactone (PCL) bioscaffold for bone transplant.
ACTIVITY 6: Printability of HIPE solutions (in collaboration with Prof. G. Massolini, Chemical Dept. of UniPV). To use Cellink INKREDIBLE+ for printing synthetic polymers, realized using high internal phase emulsion (HIPE) templating technique to prepare water-in-oil emulsions from a hydrophobic photopolymer, surfactant and water.
Methods
CellInk INKREDIBLE+
Pneumatic-based extrusion 3D bioprinter with dual heated print heads an UV LED curing system (365 nm, 205 nm) for crosslinking the 3D printed constructs. The bioplotter is equipped with a patented Clean Chamber Technology and HEPA fitered positive air pressure inside the printing chamber. Bioprinting process works through a layer-by-layer deposition of bioink or hydrogel with a viscosity range from 0,001 to 250 PaS (11 to 250.000 cP). Positioning precision for X and Y axes is 10 µm and for Z axis is 2,5 µm, while layers resolution is 100 µm.
Printing Protocol
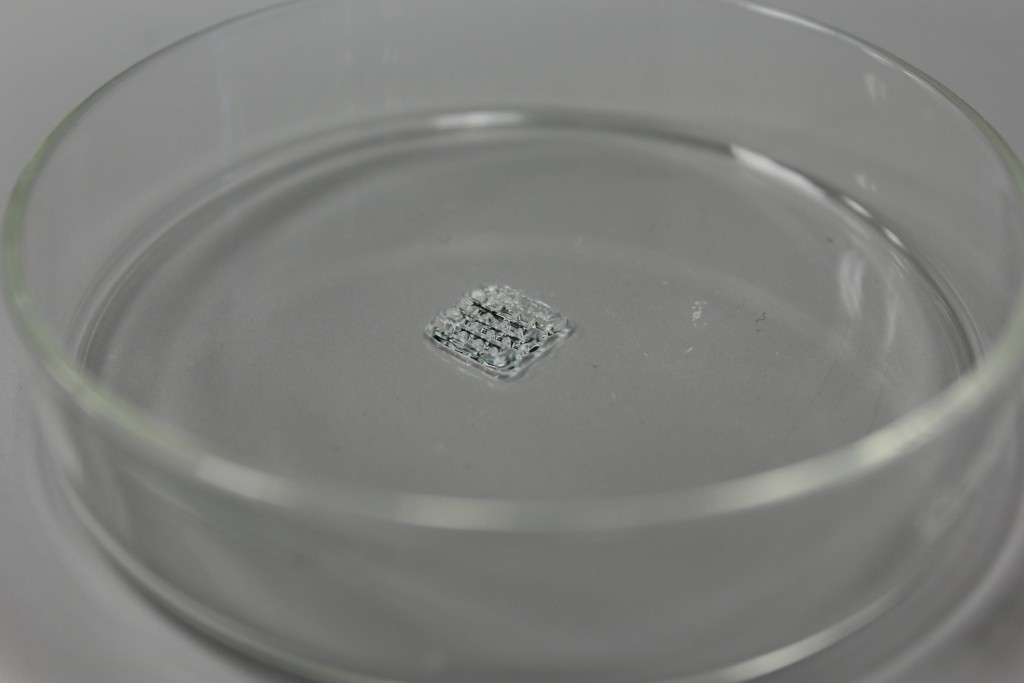
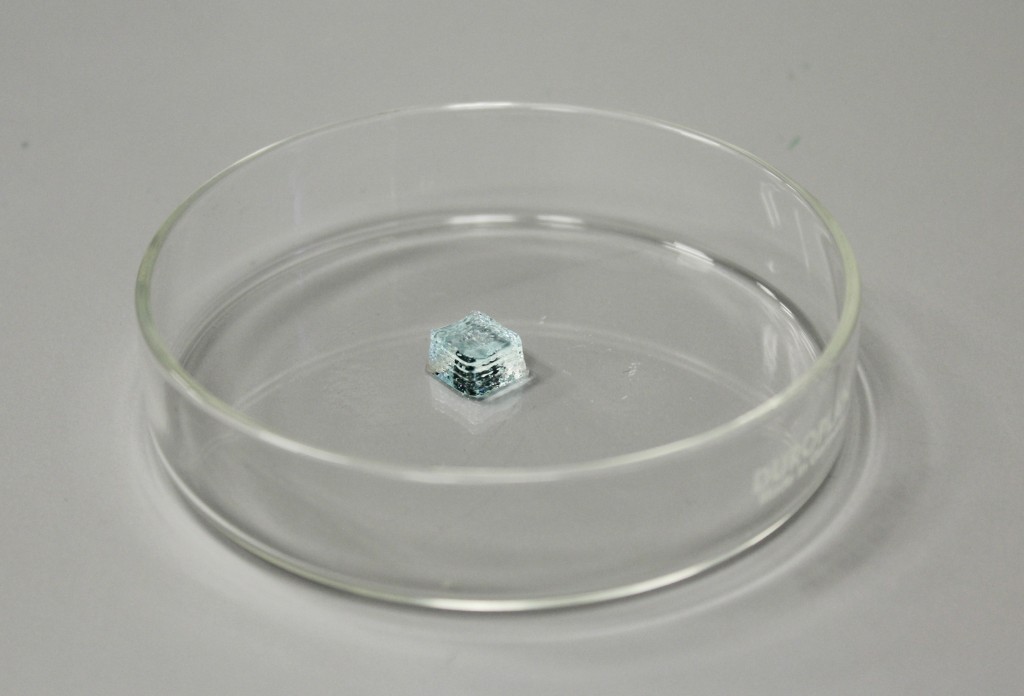
Turn on HEPA filter and set 100% air flow. Set both printheads to 37° C, optimal for cells. Connect the nozzle to the cartridges. Connect cartridges to the pressurizer. Place the cartridges in the extruder housing and check manually the correct alignment of the two extruders. Pressure calibration to zero: increase the pressure until a value is reached to allow continuous extrusion. Extrude a small amount of material to check that there are no obstructions inside the nozzle and homing extruders. Place the petri on which the sample will be extruded. Z calibration: nozzle must adhere to the petri dish. Load the g-code and start printing.
References
- A. Skardal and A. Atala. Biomaterials for integration with 3D bioprinting. Annals of Biomedical Engineering, 2015.
Collaborations
- ProtoLab, UniPv (Department of Civil Engineering and Architecture)
- Prof. G. Cusella, Doc. G. Ceccarelli and Doc. F. Ronzoni, Anatomy Department, University of Pavia
- Prof. C. Cereda, IRCCS Istituto Neurologico C. Mondino, Pavia
- Prof B. Conti, Prof. D. Pasini, Chemical Department, University of Pavia
- Doc. C. Ferrari, Doc. L. Consolino – Experimental Surgery Laboratory, University of Pavia.
- Fondazione IRCCS Policlinico San Matteo, Pavia
- Prof. G. Massolini, Chemical Department, University of Pavia